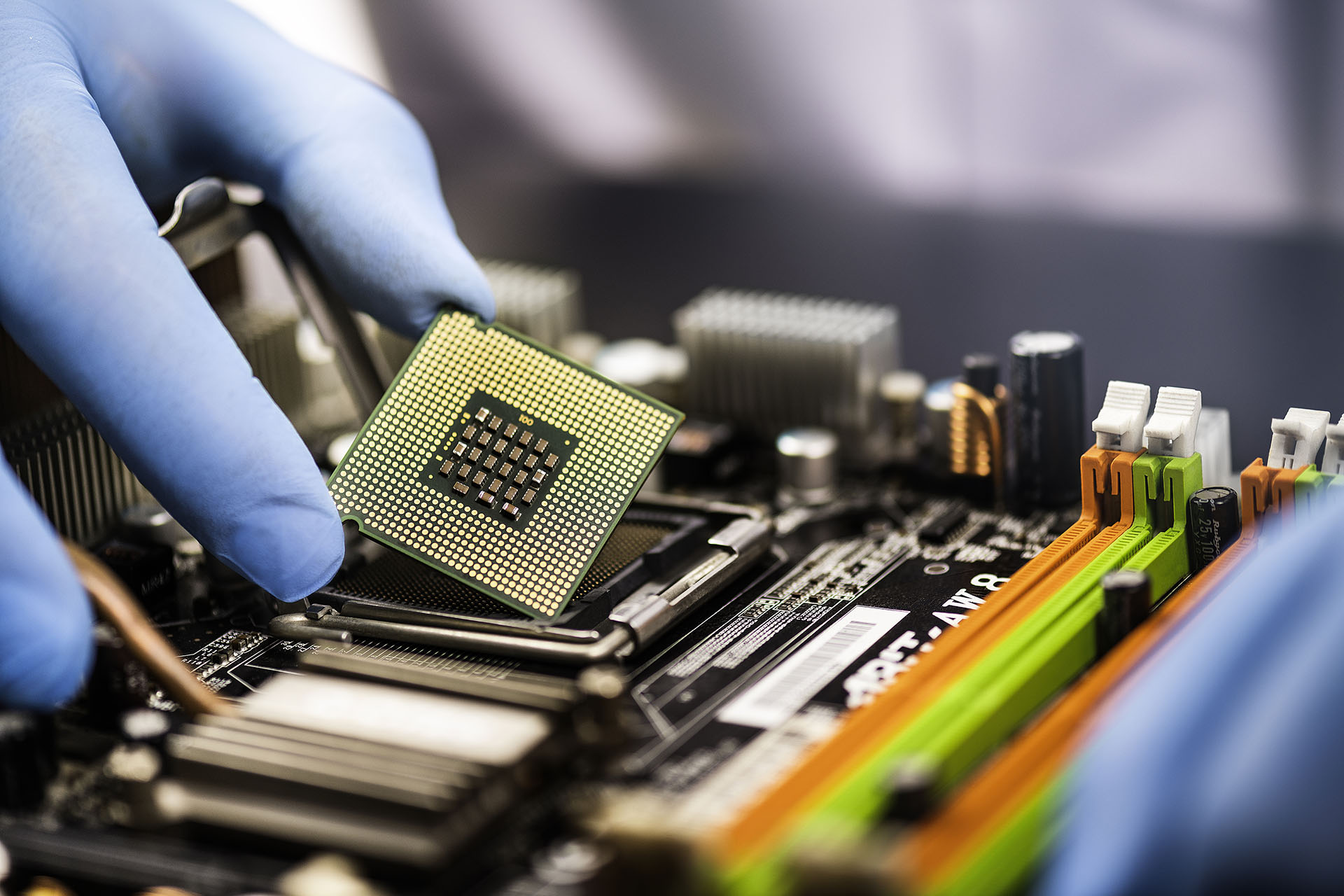
semiconductor, any of a class of crystalline solids intermediate in electrical conductivity between a conductor and an insulator. Semiconductors are employed in the manufacture of various kinds of electronic devices, including diodes, transistors, and integrated circuits. Such devices have found wide application because of their compactness, reliability, power efficiency, and low cost. As discrete components, they have found use in power devices, optical sensors, and light emitters, including solid-state lasers. They have a wide range of current- and voltage-handling capabilities and, more important, lend themselves to integration into complex but readily manufacturable microelectronic circuits. They are, and will be in the foreseeable future, the key elements for the majority of electronic systems, serving communications, signal processing, computing, and control applications in both the consumer and industrial markets.
What is a Semiconductor?
A semiconductor is a material that has an electrical conductivity value between that of a conductor and an insulator. This means that semiconductors can conduct electricity under certain conditions but not as efficiently as conductors like copper or aluminum. The conductivity of semiconductors can be controlled by introducing impurities, a process known as doping, which enables them to function effectively in electronic circuits.
The ability to control the conductivity of semiconductors is key to their role in electronic components like transistors, diodes, and integrated circuits (ICs). These devices form the foundation of modern electronics, from smartphones and computers to medical devices and vehicles.
Semiconductor materials
Solid-state materials are commonly grouped into three classes: insulators, semiconductors, and conductors. (At low temperatures some conductors, semiconductors, and insulators may become superconductors.) The shows the conductivities σ (and the corresponding resistivities ρ = 1/σ) that are associated with some important materials in each of the three classes. Insulators, such as fused quartz and glass, have very low conductivities, on the order of 10−18 to 10−10 siemens per centimetre; and conductors, such as aluminum, have high conductivities, typically from 104 to 106 siemens per centimetre. The conductivities of semiconductors are between these extremes and are generally sensitive to temperature, illumination, magnetic fields, and minute amounts of impurity atoms. For example, the addition of about 10 atoms of boron (known as a dopant) per million atoms of silicon can increase its electrical conductivity a thousandfold (partially accounting for the wide variability shown in the preceding figure).
The study of semiconductor materials began in the early 19th century. The elemental semiconductors are those composed of single species of atoms, such as silicon (Si), germanium (Ge), and tin (Sn) in column IV and selenium (Se) and tellurium (Te) in column VI of the periodic table. There are, however, numerous compound semiconductors, which are composed of two or more elements. Gallium arsenide (GaAs), for example, is a binary III-V compound, which is a combination of gallium (Ga) from column III and arsenic (As) from column V. Ternary compounds can be formed by elements from three different columns—for instance, mercury indium telluride (HgIn2Te4), a II-III-VI compound. They also can be formed by elements from two columns, such as aluminum gallium arsenide (AlxGa1 − xAs), which is a ternary III-V compound, where both Al and Ga are from column III and the subscript x is related to the composition of the two elements from 100 percent Al (x = 1) to 100 percent Ga (x = 0). Pure silicon is the most important material for integrated circuit applications, and III-V binary and ternary compounds are most significant for light emission.
Prior to the invention of the bipolar transistor in 1947, semiconductors were used only as two-terminal devices, such as rectifiers and photodiodes. During the early 1950s germanium was the major semiconductor material. However, it proved unsuitable for many applications, because devices made of the material exhibited high leakage currents at only moderately elevated temperatures. Since the early 1960s silicon has become by far the most widely used semiconductor, virtually supplanting germanium as a material for device fabrication. The main reasons for this are twofold: (1) silicon devices exhibit much lower leakage currents, and (2) silicon dioxide (SiO2), which is a high-quality insulator, is easy to incorporate as part of a silicon-based device. Thus, silicon technology has become very advanced and pervasive, with silicon devices constituting more than 95 percent of all semiconductor products sold worldwide.
Many of the compound semiconductors have some specific electrical and optical properties that are superior to their counterparts in silicon. These semiconductors, especially gallium arsenide, are used mainly for optoelectronic and certain radio frequency (RF) applications.
Examples of Semiconductors
Some of the most common semiconductors used in electronic devices include:
• Silicon (Si): The most widely used semiconductor material, silicon is the primary element in the production of integrated circuits (ICs) and transistors. Its abundance, cost-effectiveness, and stable properties at room temperature make it ideal for use in the vast majority of electronic devices.
• Germanium (Ge): Germanium was one of the first semiconductors used in electronic components. Although it has largely been replaced by silicon due to its higher cost and lower performance, germanium is still used in some specialized applications, such as in infrared detectors and certain high-speed devices.
• Gallium Arsenide (GaAs): Gallium arsenide is often used in applications requiring high-speed performance, such as in microwave frequency devices, optical communication systems, and high-efficiency solar cells. GaAs has a higher electron mobility than silicon, which allows it to perform better in certain environments.
• Silicon Carbide (SiC): Silicon carbide is used in high-power, high-temperature, and high-voltage applications like power electronics and electric vehicle (EV) systems. SiC has superior thermal conductivity and can withstand much higher temperatures than silicon.
Types of Semiconductors
Semiconductors can be classified into two broad categories based on their doping:
• Intrinsic Semiconductors: These are pure semiconductors, such as silicon, that have no impurities added. In intrinsic semiconductors, the number of charge carriers (electrons or holes) is determined solely by the temperature and the material’s inherent properties.
• Extrinsic Semiconductors: These semiconductors have been doped with impurities to increase their electrical conductivity. There are two types:
n-type (negative-type): Doping with elements that have more electrons than the semiconductor material (e.g., phosphorus in silicon) creates an excess of electrons, which act as charge carriers.
p-type (positive-type): Doping with elements that have fewer electrons than the semiconductor material (e.g., boron in silicon) creates "holes," or the absence of electrons, which act as positive charge carriers.
Uses of Semiconductors
Semiconductors are used in a wide range of applications, making them one of the most important materials in modern technology. Here are some of the primary uses of semiconductors:
• Transistors: The fundamental building block of modern electronics, transistors are used to amplify or switch electronic signals. They are found in nearly every electronic device, from simple amplifiers to complex processors in computers and smartphones.
• Integrated Circuits (ICs): ICs are collections of interconnected transistors, diodes, and resistors that perform complex functions in a compact form. These are the heart of devices like computers, tablets, smartphones, and medical equipment. ICs enable powerful computing and efficient data processing.
• Solar Cells: Semiconductors like silicon are used in photovoltaic cells, which convert sunlight into electricity. This renewable energy technology relies heavily on semiconductor properties for efficient energy conversion.
• LEDs and Lasers: Light-emitting diodes (LEDs) and laser diodes are based on semiconductor materials. These devices are used in everything from display screens to optical communication systems.
• Sensors: Semiconductors are also employed in a variety of sensor technologies, including temperature sensors, motion detectors, and environmental sensors used in industrial and automotive applications.
Materials Used in Semiconductor Manufacturing
The semiconductor manufacturing process involves creating extremely thin wafers from raw materials, which are then processed and integrated into chips. The following are the most commonly used materials in semiconductor manufacturing:
• Silicon: As mentioned earlier, silicon is the most common material for semiconductor devices due to its abundance and excellent electronic properties.
• Germanium: Germanium is used in high-speed and high-frequency applications but is often more expensive and difficult to process than silicon.
• Compound Semiconductors (e.g., Gallium Arsenide): These materials offer enhanced performance over silicon in certain applications, particularly where high speed and efficiency are required, like in wireless communication systems and radar technology.
• Organic Semiconductors: In recent years, organic materials have gained attention for use in flexible electronics, OLED displays, and other novel technologies. These materials are potentially cheaper and more versatile than traditional semiconductors.
Devices Enabled by Semiconductors
The widespread use of semiconductors has led to the development of a vast array of electronic devices. Some of the most notable include:
• Computers and Laptops: Modern computing devices rely on semiconductor processors (CPUs) and memory chips to perform billions of calculations per second. These chips are made using advanced semiconductor manufacturing techniques to achieve incredible processing power and efficiency.
• Smartphones and Tablets: These portable devices are powered by semiconductors, with chips that handle everything from processing and memory to communication and sensor functions. The evolution of mobile processors has enabled faster, more powerful devices.
• Telecommunications Equipment: The semiconductor industry powers everything from cell towers and routers to optical fiber communication systems. Semiconductors enable efficient transmission of data over long distances, supporting the global internet infrastructure.
• Automotive Industry: With the rise of electric vehicles (EVs) and autonomous driving technologies, semiconductors are playing an increasing role in automotive systems. Power electronics, sensors, and processing units rely heavily on semiconductor components.
Fun Facts About Semiconductors
• Silicon Valley’s Name: The iconic "Silicon Valley" in California is named after the large number of companies that use silicon chips to manufacture their electronic products.
• Miniaturization: The continual miniaturization of semiconductor components, known as Moore’s Law, has driven the development of increasingly powerful and compact electronic devices. As transistor sizes shrink, their performance increases, leading to faster processors and more efficient chips.
• Quantum Computing: Researchers are exploring new semiconductor materials and architectures for quantum computing, which promises to revolutionize computing by solving problems that are currently too complex for classical computers.
Conclusion
Semiconductors are undeniably one of the most transformative materials in modern technology. From the smartphones in our pockets to the advanced medical devices that save lives, semiconductors enable the devices and systems that make our lives better, faster, and more connected. The continued innovation in semiconductor materials, devices, and manufacturing processes will drive the next wave of technological advancements, creating exciting possibilities for the future.













